Production anticipée des intrathécales en pédiatrie : questionnaire national des pratiques actuelles
23 novembre 2020
L. GUIRLE1 , M.S. PIDOUX1, J. HELOURY1, B. DESSANE1, A. VENET1, V. SERVANT1, S. CRAUSTE-MANCIET1,2 1 Unité de pharmacotechnie, Centre Hospitalier Universitaire de Bordeaux, France2 ARNA ChemBioPharm U1212 INSERM – UMR 5320 CNRS, Université de Bordeaux, France
Objective
Intrathecals (IT) are currently prepared in our hospital pharmaceutical technology department with stability set to 8 hours. The aim of our study was to evaluate current practices of production and storage of pediatrics’ ITs in hospital pharmacies to envision anticipated preparation i.e. for the week-end.
Material/Methods
National survey was sent to 41 hospital pharmacies in France and in Switzerland by email. Production environment, preparation conditions, equipment used, storage conditions, shelf life of the preparation, internal stability study conducted were the items contained in the survey.
Results- Discussion
30 answers were collected which represent 73% of participation.
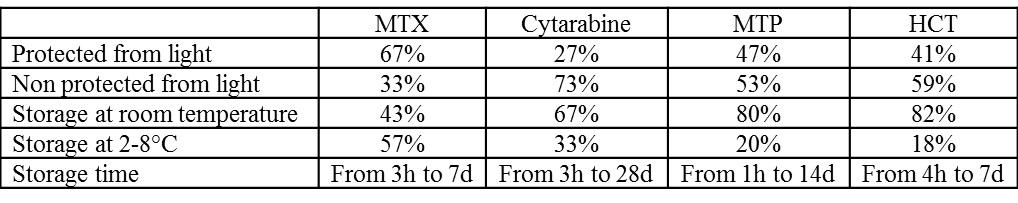
Results obtained are given in Table I
Table I. Comparison of practices for ITs preparation
Only 2 hospital pharmacies conducted in-house microbiological stability studies. Our survey demonstrated a major heterogeneity of current practices in the different center with no real support of published studies except two papers1,2.
Conclusion
These results encouraged us to conduct additional stability studies i.e. microbiological assessment to support anticipation of IT production and storage.
(1) G. Binson, L. Danguy des Déserts, A. Bousseau, A.-C. De Boisgrollier, I. Princet – Determination of the microbiological stability of intrathecal chemotherapy 18th GERPAC conference ; 2018.
(2) C. Berge Bouchara – Etude de la stabilité microbiologique de préparations de chimiothérapie pour administration intrathécale en pédiatrie (Thèse). Rouen (France) : Université de Rouen Normandie ; 2014.