Capability analysis of a powder for filling by an automatic hard capsule filler
2 October 2024
A. Melaye, N. Thomasset, M. Decuyper, L. Pacqueu, M. Vasseur, M. Bouchfaa, P. OdouCentre Hospitalier Universitaire de Lille, France
Introduction et objective
Hospital compounding plays a crucial role in offering alternatives to drugs in supply shortage. In order to achieve rapid, large-scale production in the face of rising demand, manufacturing automation appears to be a solution. The aim of this study is to determine the process capability with different excipients used as a basis for future preparations using an automatic hard capsule filler.
Materials and methods
Ten 200g powders with microcrystalline cellulose (Cooper) as the main excipient were tested: “cellulose without flow agent” (1), “anhydrous colloidal silica (SiO2) (Inresa) 0.7% (2), 1% (3), 1.2% (4), 1.3% (5)”, “talc (Cooper) 5%” (6), “SiO2 1% + talc 5%” (7), “magnesium stearate (stearate) (Inresa) 2.5% (8), 3.5% (9)” et “stearate 2.5% + SiO2 1%” (10).
Each mixture was blended using a three-dimensional blender (Inversina 2L, Bioengineering) at speed 8/10 for 30 minutes. Powders containing silica were sieved before mixing.
For each powder, 300 capsules of size 0, divided into sub-batches of 30 units, are sampled continuously during production. Hard capsule are manufactured using an automatic capsule filler (IN-CAP SE, Bonapace) and weighed. Production took place at 18°C +/- 1 and a humidity level of 56.5% +/- 3.75.
Control charts were produced using R software (qcc library; version 2.7) from the last 10 capsules of each sub-batch. The software calculates the capability (Cp), the deviation index (Cpk) and the Taguchi index (Cpm) from the total production.
Results
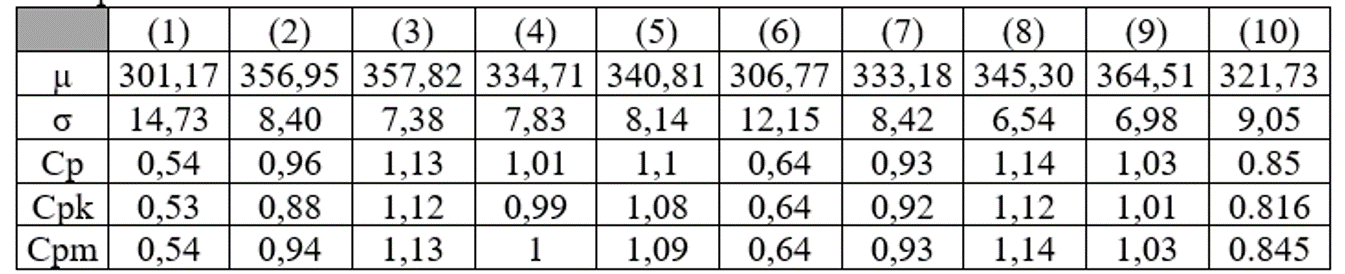
For each powder, the mean weights (µ in mg), mean standard deviations (σ in mg) and capability indices are:
According to the interpretation of the capability indicators, (1) and (6) are very inadequate, the process is out of control (Cp < 0.67). (2), (7) and (10) are insufficient, requiring 100% control of the units produced (Cp < 1). (3), (4), (5), (8), and (9) are in a delicate situation where the process can lead to errors (Cp < 1.33).
Discussion
The control charts show large variations in weight between powders. No formulation achieves optimal process control, but formulations (3) and (8) are acceptable. Regular sampling during production is still required to validate the process. Capability tests with a modified cellulose specific to automated production from the pharmaceutical industry and mixture dissolution tests should be carried out.