3D printing as a way towards new galenic forms: formulation and control of gummies containing Amiodarone or Diazoxide
2 October 2024
I. Killisly, A. Gillette, S. Crauste-Manciet, F. Lagarce, S. Vrignaud, V. LebretonService de Pharmacie, CHU d’Angers, France
Implementation of innovative technologies, such as 3D printing of medicines, represents a major and promising advance in personalized medicine, with the development of new-generation galenic formulations.
The technology developed by CurifyLabs is based on the extrusion of a biocompatible ink (Curablend®) at 45°C to form gummies. The active ingredient is then mixed with the Curablend®. In order to assess the drug potential of this new technology, gummies containing amiodarone or diazoxide were formulated. These 2 active ingredients were chosen because they have very distinct physico-chemical characteristics, particularly in terms of molar weight (MW), log P and solubility in water.
(MWAmiodarone= 645 g/mol ; Log PAmiodarone= 7.2 / MWDiazoxide=230 g/mol ; Log PDiazoxide= 1.20).
Objective
The aim of this study is to print gummies containing amiodarone or diazoxide, then to carry out controls in accordance with the requirements of the European Pharmacopoeia (EP) in order to certify their conformity and therefore their possible use in humans (pharmaceutical forms: tablets).
Materials & methods
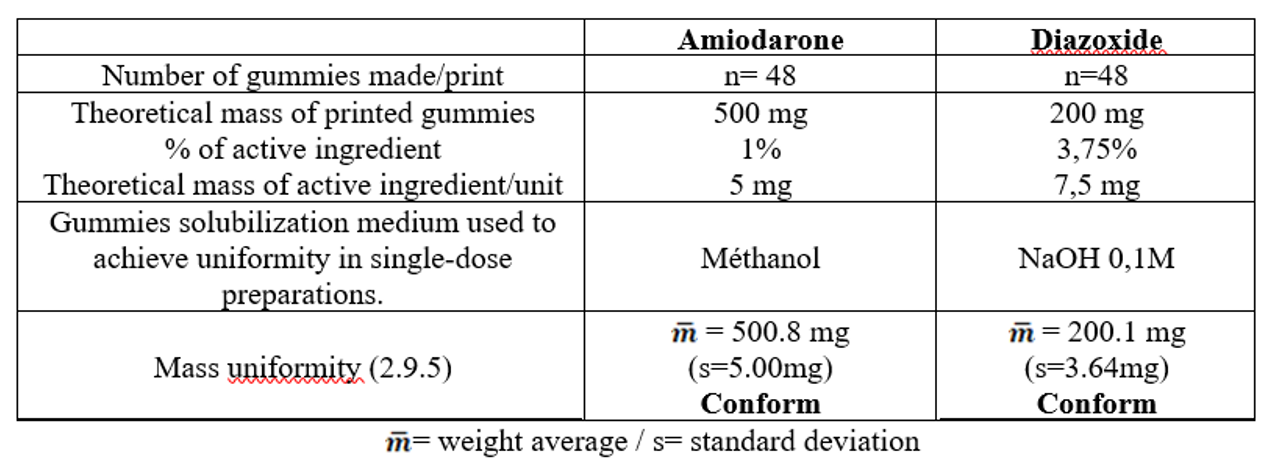
* Development of formulations for each of the molecules and printing of batches of gummies in triplicates (Pharma Printer 1, CurifyLabs, Helsinki, Finland).
* In-process mass uniformity control (Pharma Printer 1, CurifyLabs, Helsinki, Finland).
* Uniformity control of single-dose preparations (PE 2.9.40) carried out using a UV-Visible spectrophotometer (Emc², SAFAS, Monaco) with methods previously validated in accordance with ICH Q2R1 according to the recommendations of the PE for wavelengths (λmax) and solubilization media
Results
Conclusion
This study shows that the ink enables uniform mixing and therefore homogeneous printing on 2 active ingredients with two different solubility profiles, one more hydrophilic (diazoxide) and the other lipophilic (amiodarone), offering the prospect of strong development potential for a wide range of active ingredients.
In order to complete this study and to meet the release controls required by the EP, it would be interesting to develop a dissolution test (EP 2.9.3) using the produced preparations.